Pressure vs. Volume:
Constant: Temperature
Boyle's Law
What We Did: For this experiment we wanted to test what happened to the volume of air in a syringe as we added more pressure to it. So we used the handy dandy chemistry book in Mr. Ludwig's classroom to add more pressure. As you can see, as we added more books the volume of air steadily went down. We started with 50 mL and by the time we ended the experiment with seven books worth the pressure on the syringe we were down to 12 mL. Our experiment proves Boyle's Law which states that as Pressure goes up the Volume will go down.
Pressure vs. Temperature:
Constant: Volume
Guy-Lussac's Law
What We Did:
In this lab we were trying to display what happens to the pressure of air in a syringe as the temperature surrounding the syringe increases or decreases. So we used a temperature probe to help keep track of the temps we were putting the syringe in. We tested the syringe in a hot but not too hot water, a hot water, ice and then also at room temperature. As you can see by the different points on the graph, the higher the temperature the higher the pressure of the air in the syringe. Our experiment therefore proves Guy-Lussac's Law, that as temperature goes up so does pressure.
Volume vs. Temperature:
Constant: Pressure
Charles Law
For some reason I do not have a graph for this part of the experiment but! I will do my best to explain what the point of it was. The point was to test how Volume reacts under different temperatures.But even though I don't have information on this part of the lab I can tell you that, Charles Law states that as Temperature goes up, Volume will also go up.
I know this because...
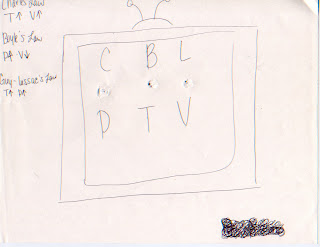
Below is an activity that Mr. Ludwig has us to do to learn more about the gas laws. If you look closely you can see the holes I poked through the paper. First, you stick your pencil through on of the holes. As you turn it, it will show you how Temp and Volume relate in Charles Law, Pressure and Volume relate in Boyle's Law and Temperature and Pressure relate in Guy-Lussac's Law.